依據歐盟施行的個人資料保護法,我們致力於保護您的個人資料並提供您對個人資料的掌握。
按一下「全部接受」,代表您允許我們置放 Cookie 來提升您在本網站上的使用體驗、協助我們分析網站效能和使用狀況,以及讓我們投放相關聯的行銷內容。您可以在下方管理 Cookie 設定。 按一下「確認」即代表您同意採用目前的設定。
About Meribank Biotech co.
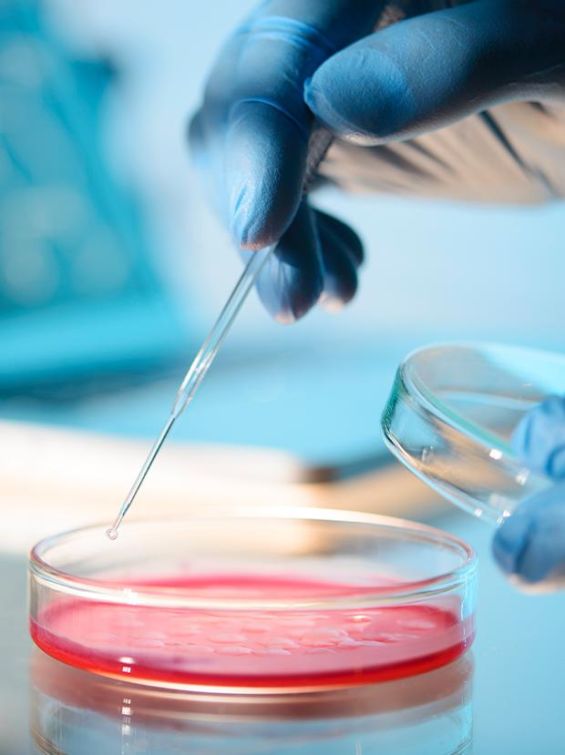
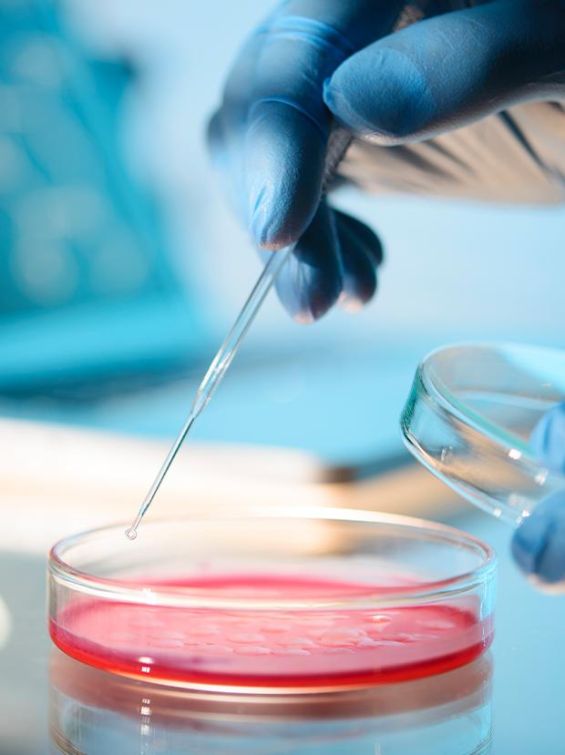
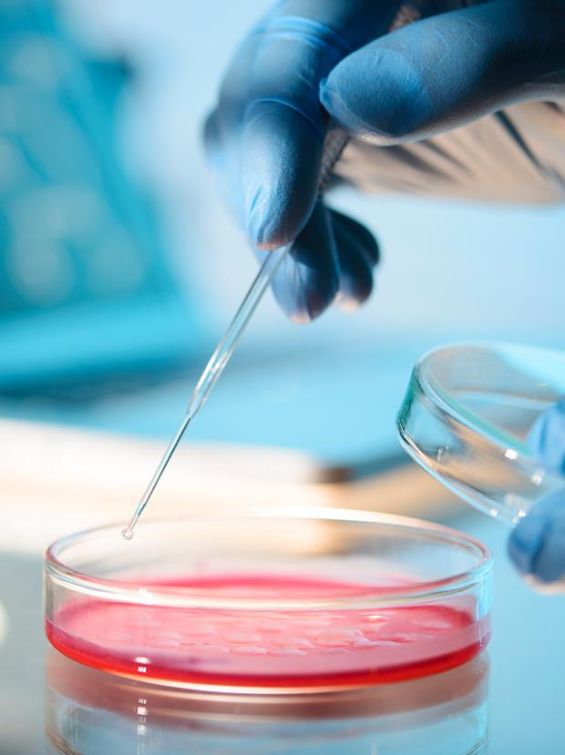
Meribank Biotech co. is dedicated to developing mesenchymal stem cells(MSCs)-based technologies from manufacturing, characterization, storage, to drug discoveries. Moreover, we have been acquiring other avant-garde technologies, such as machine learning and artificial intelligence, to strive toward invention of smart medical products for the improvement of innovation and precision of medicine. Meridigen’s MSC drugs strictly follow the international standard of Pharmaceutical Inspection Co-operation Scheme Good Manufacturing Practice(PIC/S GMP). We also can produce Exosome with the same specifications.




Stem Cell Research & Storage Center
Strictly examined by the US FDA Stem Cell Drug Chemistry Manufacturing Control(CMC). In accordance with the PIC/S GMP international pharmaceutical grade specification, following US FDA Cellular Pharmaceutical Specification(CMC) for stem cell storage, using advanced equipment from the US FDA to perform stem cell testing and cryopreservation. The progress of cell drugs has entered the Phace II clinical trial.

Meridigen Group Exclusive Patent Technology
As a leading brand, Meridigen Group introduces US cell culture technology to purify and expand placenta and umbilical cord. P1 primary stem cells can be cultured from “50 million” to “300 million”. Significantly reduce the probability of cell variability, strictly control and enrich each mesenchymal stem cell, making it more widely available in the future, and can be used by family members. We can provide high stability and high quality MSC's Exosome.